It is anticipated that community pharmacies will experience a greater demand this year for pharmacist-administered vaccinations because of COVID-19 concerns and the reduction in the minimum age for influenza vaccination in many States. PDL is concerned that an increased number of virus-related enquiries and higher demand for vaccination when many pharmacies are dealing with larger-than-normal volumes of prescriptions may increase the risk of an incident.
PDL would like to remind all pharmacists and pharmacy owners to ensure appropriate vaccination training, qualifications and procedures are reviewed and updated. Pharmacies undertaking vaccination should be adequately staffed which will include ancillary staff who are trained in CPR and first aid. Current first aid qualifications are vital for pharmacists, and certification should be sighted or recorded. A suitable area for vaccination must comply with State legislation and guidelines and include adrenaline with clearly noted expiry dates and reminders for replacement prior to expiry. State regulators can undertake inspections of pharmacy vaccination services with one specific example being the recent announcement from NSW Health regarding commencement of random vaccination audits in that State. Please review your local Health Department websites for further information regarding vaccination standards.
The Australian Technical Advisory Group on Immunisation (ATAGI) have released their 2020 statement outlining the age ranges for influenza vaccines in Australia. As shown in the ATAGI table below, the age limits for some of these brands have changed since 2019. Also, Fluad Quad is a newly approved adjuvanted quadrivalent vaccine specifically for patients 65 years and above.
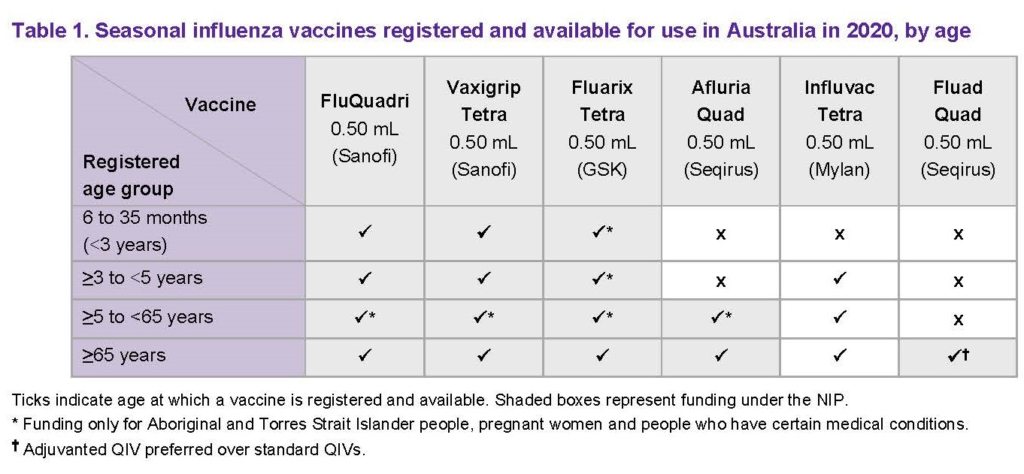
Review your State Health Department’s website for the minimum age limits and range of vaccines permitted for pharmacist vaccination as it’s quite likely that these details have changed since the 2019 season. At the time of publishing recent changes have been made to legislation in Qld, NSW, Victoria and SA.
While vaccine-related incident reports to PDL in 2019 were very small in proportion to the number of vaccines administered, there were a few consistent incident types. These included shoulder injuries following vaccination, age-related issues and incidents post-vaccination.
Shoulder injury related to vaccine administration (SIRVA) is a complication of vaccination by any health professional and a very small number of reports were received by PDL. Good administration technique as per training, pharmacist positioning in relation to patient i.e. sitting rather than standing and thorough assessment of the intended site of vaccination can help prevent this complication. A patient presenting with persistent pain following vaccination should be advised to seek medical advice. The Australian Immunisation Handbook has a document assisting practitioners to address this issue.
Age related issues included patients under the minimum age being inadvertently vaccinated by a pharmacist through oversight or misleading information from the patient or parent. The greater demand for vaccination in conjunction with Covid-19 concerns may see greater presentation of underage patients and good procedures including review of the consent form before administration will be of assistance in such cases. Pharmacists are reminded that generically written prescriptions for influenza vaccines for children must be supplied with a vaccine approved for that age group. PDL suggests that vaccines are stored with a note or laminated message identifying the age range of each brand of vaccine to prevent error. Good procedures by pharmacy staff such as confirming the age of patients with a prescription for vaccines is encouraged. PDL is aware of incidents when a patient over 65 years has received a pharmacist-administered vaccine that was not adjuvanted. While the advice is for patients in this age group to receive the adjuvanted vaccine, limitations in supply or imminent travel may warrant vaccination with another form of influenza vaccine. PDL recommends the patient be made aware of the indications and advice of regulatory and advisory bodies. Also consider contacting the prescriber and making them aware of the situation. Ensure the patient is fully informed, consents to another vaccine and there is documentation to support this action.
Incidents post-vaccination are rare, but a common theme included patients experiencing light-headedness or fainting during or shortly after vaccination. This also occurred when patients attempted to stand or leave the pharmacy too soon after vaccination and experienced unsteadiness resulting in a fall. It’s likely that most of these reactions were vasovagal episodes and pharmacists should be familiar with symptoms and management. Pharmacists and staff also need to be able to differentiate between a vasovagal episode and an anaphylactic reaction and be prepared to respond promptly and professionally. The Immunisation Handbook has resources to assist with differentiating between these conditions.
Many States now require that pharmacist-administered vaccinations be recorded in the Australian Immunisation Register (AIR). Pharmacies should check if this is a requirement in their State as it has changed recently in some jurisdictions.
Call 1300 854 838 if this topic raises any concerns for you. PDL membership includes 24/7 access to speak with a Professional Officer for immediate advice and incident support, Australia-wide. Please leave a comment or question for our Professional Officers via the blog.