The prescribing and supply of medicinal cannabis is a rapidly expanding area of practice in Australia. With this emerging field comes the need for comprehensive risk management considerations and strategies to ensure patient safety and regulatory compliance. The following explores some essential risk management considerations for pharmacists who are involved in this area of practice.
Scope of practice
Scope of practice refers to the boundaries and limitations within which an individual pharmacist practises, based on their education, competence and authority, whilst having accountability for their actions. Being clear of one’s scope can prevent individuals from performing tasks beyond their training and expertise. PDL would like to encourage pharmacists dispensing medicinal cannabis to ensure they are equipped with the knowledge and skills to fulfil their responsibilities competently and legally.
Regulatory compliance
The regulatory landscape around the manufacturing, prescribing and dispensing of medicinal cannabis is currently extremely complex. Ensuring compliance with the Therapeutic Goods Administration (TGA) guidelines and state and territory legislation is of utmost importance. In order for pharmacists to safely and legally perform their role, it is important to understand the medicinal cannabis process, from supply to prescribing and dispensing.
Due to the rapid growth in the number of medicinal cannabis suppliers and prescribers, there have been occasions where pharmacists have been provided inaccurate advice, which conflicts with that provided by regulatory agencies. Pharmacists are encouraged to check with regulators if they feel the advice provided does not align with their understanding and interpretation of relevant regulation.
Storage
Many forms of medicinal cannabis are Schedule 8 drugs and must be stored and controlled with other Schedule 8s in a secure location with appropriate controls and documentation. Regulators are currently concentrating on pharmacy compliance with storage obligations and how this is monitored in the pharmacy. It should be noted that insufficient room in the drug safe is not an adequate excuse for storing these Schedule 8 medicines outside the safe.
Prescriptions
Medicinal cannabis products can either be Schedule 4 or Schedule 8. As with all Schedule 8 medications, extra vigilance should be applied during the reviewing and dispensing process. Scripts are often issued to patients via online platforms or by telehealth, creating a challenge in contacting prescribers for clarification and discussion. Be aware that prescribers may also be linked to or aligned with medicinal cannabis suppliers.
Currently, there is a variable understanding of the legal requirements for writing prescriptions, with some prescribing software not being configured to assist with this. Be aware of the necessity for prescribers to be explicit about dosage and product substitution. Be wary of scripts that state a range of dosage strengths. Scripts must state exactly the strength of product to be supplied rather than a range.
As for all Schedule 8 medications, PDL strongly recommends the use of Real Time Prescription Monitoring (RTPM) prior to the supply of Schedule 8 medical cannabis products. This is a legal requirement in jurisdictions where RTPM is mandatory, and PDL considers it best practice in those jurisdictions where review of RTPM records is not mandatory.
Suppliers
There has been an increase in suppliers in the medicinal cannabis industry across Australia. Due to the lack of credible, high-quality information, some suppliers are creating their own guidance documents. Due to possible commercial bias, it is important to critically review any material provided to ensure that it aligns with the current information from regulatory bodies in Australia.
Recent changes
PDL would like to draw pharmacists’ attention to changes in the Therapeutics Goods Order 93 (TGO 93) that were effective from 1 July 2023. Australian-based and overseas-based manufacturers are to operate in compliance with the Good Manufacturing Practice (GMP) code. They are to ensure that manufacturing of medicinal cannabis products occur at sites that are compliant with the GMP standards. The importer (Australian sponsor) must hold evidence of the GMP compliance in accordance with section 13 of the TGO 93. While pharmacists aren’t required to verify this before dispensing a medication, pharmacists are encouraged to familiarise themselves with suppliers that meet GMP standards and/or request GMP status clarification from suppliers that approach them for product training.
Patient education
The lack of Consumer Medicine Information (CMI) for medicinal cannabis products accentuates the crucial role that pharmacists play in providing counselling and guidance to patients. This is particularly important with the potential side effect of drowsiness, with the main aim for patient education being risk minimisation. Due to the lack of information available, a higher level of patient interaction discussing dosing and timing should be made. PDL would like to remind all pharmacists that counselling and education is equally important with patients who do not attend the pharmacy in person. Ensure that documentation reflects discussions and decisions made with the patient.
It is particularly critical to highlight the implications of taking medicinal cannabis with regard to driving laws, work health and safety, and drug testing. Patients must be informed of the legal implications of driving when they may test positive for the presence of tetrahydrocannabinol (THC). PDL is aware that specific medicinal cannabis Cautionary Advisory Labels (CALs ) are now available from at least one supplier of these labels. Refer to StirlingFildes HealthCare’s list of relevant CALs.
Patient education for vaporised products should encourage the purchase and use of the Australian Register of Therapeutic Goods (ARTG) listed devices. If a patient decides not to purchase or utilise an ARTG-listed device, PDL recommends that this discussion is clearly documented.
Dried herb vaporisers
There are currently three ARTG-listed dried herb vaporisers available in Australia. These devices are the preferred method of administration for medicinal cannabis flowers. All devices contain patented hybrid heating technology for efficient extraction of the active ingredients and a cooling unit allowing the vapour to cool down ahead of inhalation, reducing potential throat or lung irritation. The following table summarises dried herb vaporisers manufactured by Storz & Bickel.
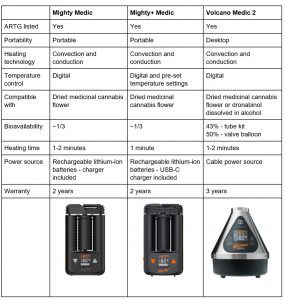
Documentation
Because of the evolving and complex nature of medicinal cannabis, PDL recommends pharmacists are particularly vigilant around documentation and record keeping. Informed consent from patients should be sought and documented comprehensively where data is limited for indication, potential side effects, interactions and unpredictable health consequences through inhalation.
Adverse event reporting
We strongly encourage pharmacists to report any adverse events from medicinal cannabis products to the TGA and prescribers. This will enable the TGA to continue to monitor the safety of these products and investigate any significant safety concerns. Through diligent reporting, pharmacists contribute to a broader culture of pharmacovigilance. Collective data contributes to the safe and effective use of these agents and can help with the refining of guidelines and practice protocols moving forward.
Incident reporting
As with any other therapeutic agent, PDL strongly advocates and encourages pharmacists to report incidents or observations relating to the supply of medicinal cannabis. This is beneficial to raise the awareness of pharmacists, prescribers and regulators.
Third party supply
As pharmacists play a pivotal role in facilitating patient access to medicinal cannabis, a robust risk management approach is essential to ensure safe, effective and compliant practices. PDL is aware of instances where pharmacists have encountered external pressures to engage in third-party supply arrangements with companies. PDL would like to remind pharmacists to prioritise patient well-being whilst upholding the integrity of the healthcare ecosystem in this area of practice.
Useful links
This is not an exhaustive list, but PDL would like to encourage pharmacists to access information available from the TGA, state and territory department websites, the Pharmaceutical Society of Australia and The University of Sydney.
- TGA – Medicinal cannabis hub
- Vic Department of Health – Medicinal cannabis regulatory framework
- Vic Department of Health – Medicinal cannabis
- NSW Health – Cannabis medicines
- Queensland Health – Medicinal cannabis
- ACT Health – Medicinal cannabis
- WA Department of Health – Cannabis-based products
- Tas Department of Health – Medicinal cannabis
- SA Health – Medicinal cannabis – Patient access in South Australia
- SA Health – Frequently asked questions on medicinal cannabis
- Access to medicinal cannabis and CBD oil in the Northern Territory
- PSA – Medicinal cannabis education resources
- The University of Sydney, Lambert Initiative for Cannabinoid Therapeutics – Medicinal cannabis
For immediate advice and incident support, call PDL on 1300 854 838 to speak with one of our Professional Officers. We are here to support our pharmacist members 24/7.



